Most fuel cells designed for use in vehicles produce less than 1.16 volts of electricity-far from enough to power a vehicle. Therefore, multiple cells must be assembled into a fuel cell stack.
The potential power generated by a fuel cell stack depends on the number and size of the individual fuel cells that comprise the stack and the surface area of the PEM.
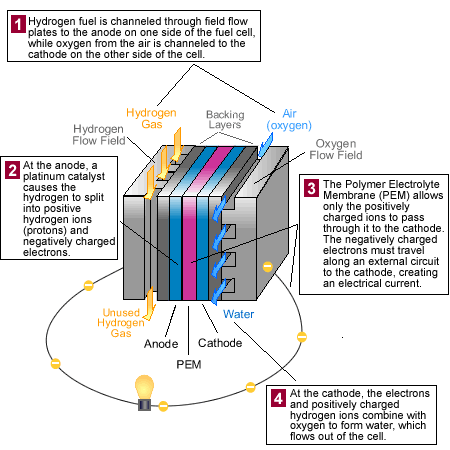
1) Hydrogen fuel is channeled through field flow plates to the anode on one side of the fuel cell, while oxygen from the air is channeled to the cathode on the other side of the cell.
2)At the anode, a platinum catalyst causes the hydrogen to split into positive hydrogen ions (protons) and negatively charged electrons.
3) The Polymer Electrolyte Membrane (PEM) allows only the positively charged ions to pass through it to the cathode. The negatively charged electrons must travel along an external circuit to the cathode, creating an electrical current.
4)At the cathode, the electrons and the positively charged hydrogen ions combine with oxygen to form water, which flows out of the cell.
The diagram below shows how these fuel cells work:
 |
| Pem Polymer Electrolyte Membrane |
U.S. Dept. of Energy